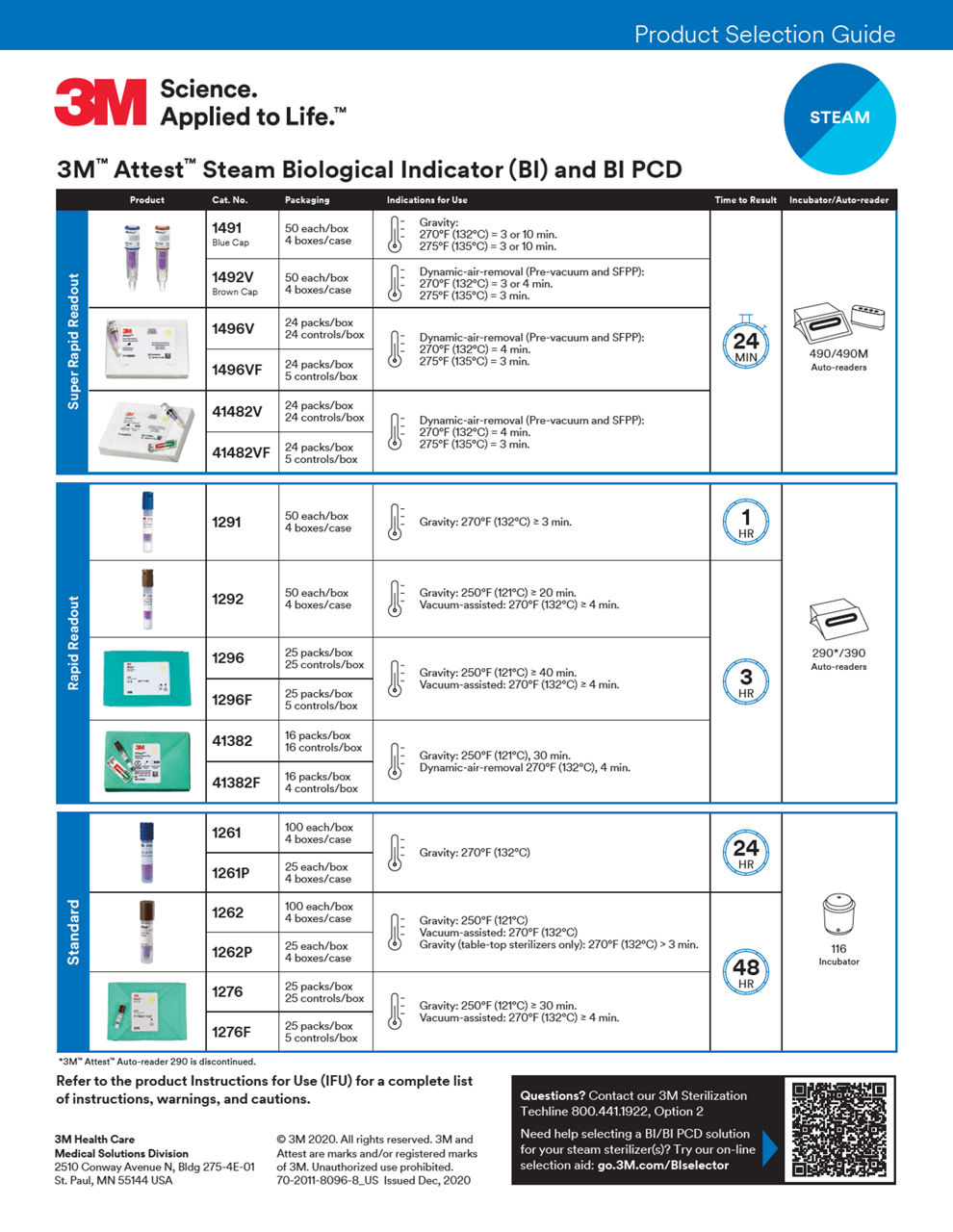
Biological indicators
How a BI works
The theory behind the BI is that if your process is effective enough to kill a large population of highly resistant spores, it will also kill a lower number of less resistant organisms on the medical devices. Because it detects the killing of microbial spores, a BI is capable of yielding information that is more valuable than any other sterilization monitor.
After exposure to the sterilization process, self-contained BIs are activated to provide the spores with optimized growth media, and then must be incubated at the appropriate temperature to determine if any spores survived. For this reason, results are not immediately available. However, advances in BI technologies have led to rapid readout BIs, which provide the end user with actionable results in less than 30 minutes.
What is sterility assurance level (SAL)?
Sterile is the state of being free of all living microorganisms and is usually expressed as a probability.1 A sterility assurance level (SAL) is a number that tells you the probability of a viable microorganism being present on an item after sterilization.1 This number expresses the likelihood that a microorganism survived the sterilization. An SAL of 10-6 (or 10 to the negative sixth power) indicates there is one chance in a million that a device is not sterile and is generally accepted as appropriate for an item intended to contact compromised tissue.1
The sterilizer manufacturer is responsible for providing a sterilizer and process that can achieve the desired SAL and uses BIs as a tool to estimate SAL and develop and validate their sterilization processes. The user is responsible for monitoring the performance of the sterilizer (e.g., with a BI) to ensure it is operating as intended1.
Latest innovations

No assembly required
3M™ Attest™ Super Rapid Vaporized Hydrogen Peroxide Clear Challenge Pack
Save time and reduce potential error with the 3M™ Attest™ Super Rapid Vaporized Hydrogen Peroxide Clear Challenge Pack including both a BI and a CI. It’s the first and only preassembled VH2O2 test pack that can be used for routine monitoring and qualification across multiple sterilizer brands, models and cycle types.*
*as of November 11, 2024
Key resources
Biological Indicators
Select Filters to narrow your product list
1 - 9 of 20 Products
References:
- CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines-H.pdf [accessed June 27, 2023].
- ANSI/AAMI ST79:2017 Comprehensive guide to steam sterilization and sterility assurance in health care facilities.